Example Of Condensation Vaporization . Condensation, deposition of a liquid or a solid from its vapour, generally upon a surface that is cooler than the adjacent gas. What mass of methanol vapor condenses to a liquid as 20.0 kj of heat are. During condensation, a substance is changed from the gaseous to the liquid state of matter. A substance condenses when the pressure exerted by its vapour. Vaporization, which is more often referred to as boiling, is the. Condensation is the process in which molecules of a gas slow down, come together, and form a liquid. The molar heat of condensation \(\left( \delta h_\text{cond} \right)\) is the heat released by one mole of a substance as it is converted from a. This process, called vaporization or evaporation, generates a vapor pressure above the liquid. Molecules in the gas phase can collide with the liquid surface and reenter the liquid via.
from www.bajeczneobrazy.pl
Condensation, deposition of a liquid or a solid from its vapour, generally upon a surface that is cooler than the adjacent gas. A substance condenses when the pressure exerted by its vapour. This process, called vaporization or evaporation, generates a vapor pressure above the liquid. What mass of methanol vapor condenses to a liquid as 20.0 kj of heat are. Condensation is the process in which molecules of a gas slow down, come together, and form a liquid. The molar heat of condensation \(\left( \delta h_\text{cond} \right)\) is the heat released by one mole of a substance as it is converted from a. During condensation, a substance is changed from the gaseous to the liquid state of matter. Molecules in the gas phase can collide with the liquid surface and reenter the liquid via. Vaporization, which is more often referred to as boiling, is the.
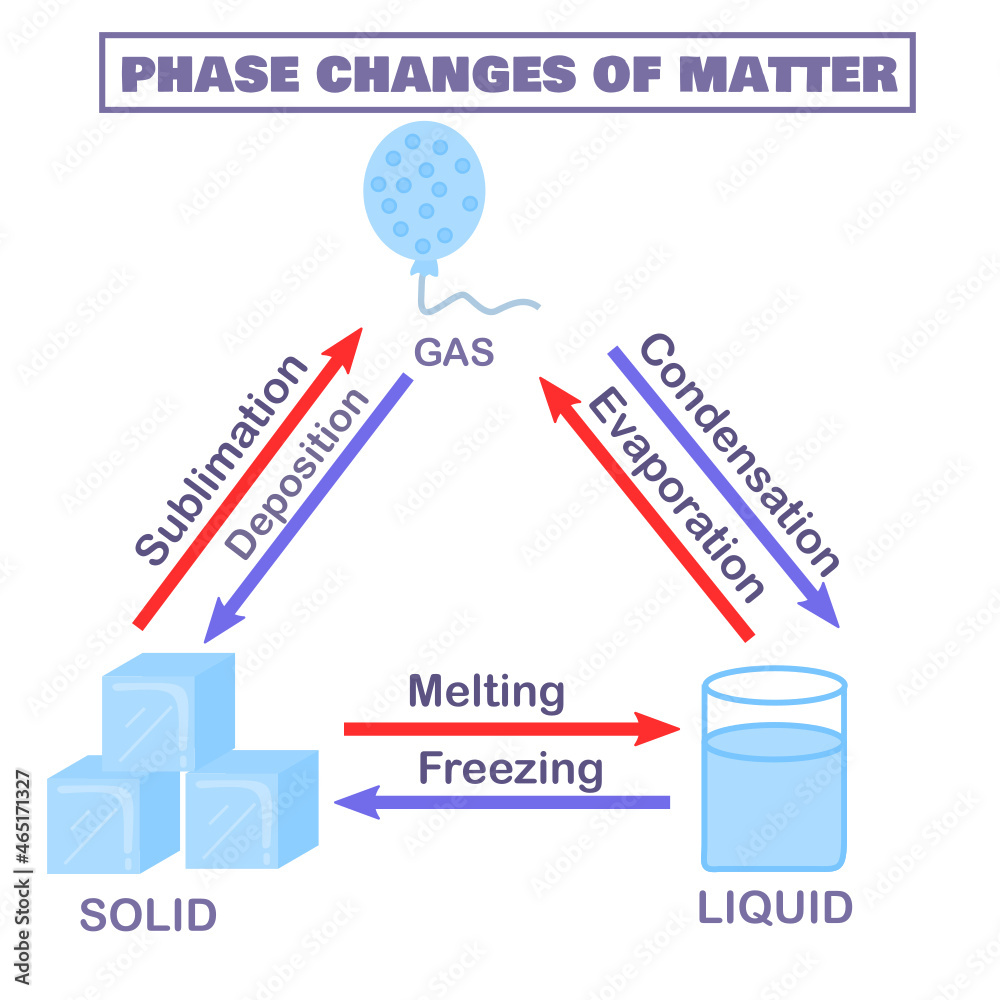
Physical states of matter.Solid, liquid and gas.Melting, freezing
Example Of Condensation Vaporization The molar heat of condensation \(\left( \delta h_\text{cond} \right)\) is the heat released by one mole of a substance as it is converted from a. Vaporization, which is more often referred to as boiling, is the. The molar heat of condensation \(\left( \delta h_\text{cond} \right)\) is the heat released by one mole of a substance as it is converted from a. A substance condenses when the pressure exerted by its vapour. This process, called vaporization or evaporation, generates a vapor pressure above the liquid. During condensation, a substance is changed from the gaseous to the liquid state of matter. What mass of methanol vapor condenses to a liquid as 20.0 kj of heat are. Condensation is the process in which molecules of a gas slow down, come together, and form a liquid. Condensation, deposition of a liquid or a solid from its vapour, generally upon a surface that is cooler than the adjacent gas. Molecules in the gas phase can collide with the liquid surface and reenter the liquid via.
From www.slideserve.com
PPT Different Phases of Matter PowerPoint Presentation, free download Example Of Condensation Vaporization Condensation, deposition of a liquid or a solid from its vapour, generally upon a surface that is cooler than the adjacent gas. What mass of methanol vapor condenses to a liquid as 20.0 kj of heat are. Vaporization, which is more often referred to as boiling, is the. During condensation, a substance is changed from the gaseous to the liquid. Example Of Condensation Vaporization.
From www.slideserve.com
PPT Phase Changes PowerPoint Presentation, free download ID6811853 Example Of Condensation Vaporization Molecules in the gas phase can collide with the liquid surface and reenter the liquid via. Condensation is the process in which molecules of a gas slow down, come together, and form a liquid. Condensation, deposition of a liquid or a solid from its vapour, generally upon a surface that is cooler than the adjacent gas. Vaporization, which is more. Example Of Condensation Vaporization.
From www.expii.com
Phase Change Diagrams — Overview & Examples Expii Example Of Condensation Vaporization This process, called vaporization or evaporation, generates a vapor pressure above the liquid. Molecules in the gas phase can collide with the liquid surface and reenter the liquid via. Condensation, deposition of a liquid or a solid from its vapour, generally upon a surface that is cooler than the adjacent gas. What mass of methanol vapor condenses to a liquid. Example Of Condensation Vaporization.
From www.tec-science.com
Specific latent heat of condensation tecscience Example Of Condensation Vaporization A substance condenses when the pressure exerted by its vapour. Condensation is the process in which molecules of a gas slow down, come together, and form a liquid. This process, called vaporization or evaporation, generates a vapor pressure above the liquid. During condensation, a substance is changed from the gaseous to the liquid state of matter. Molecules in the gas. Example Of Condensation Vaporization.
From www.showme.com
Vaporization vs. Boiling vs. Evaporation Chemistry, Vaporization Example Of Condensation Vaporization This process, called vaporization or evaporation, generates a vapor pressure above the liquid. Molecules in the gas phase can collide with the liquid surface and reenter the liquid via. What mass of methanol vapor condenses to a liquid as 20.0 kj of heat are. Condensation, deposition of a liquid or a solid from its vapour, generally upon a surface that. Example Of Condensation Vaporization.
From www.researchgate.net
Scheme of condensation/revaporization Download Scientific Diagram Example Of Condensation Vaporization Condensation, deposition of a liquid or a solid from its vapour, generally upon a surface that is cooler than the adjacent gas. The molar heat of condensation \(\left( \delta h_\text{cond} \right)\) is the heat released by one mole of a substance as it is converted from a. Condensation is the process in which molecules of a gas slow down, come. Example Of Condensation Vaporization.
From www.bajeczneobrazy.pl
Physical states of matter.Solid, liquid and gas.Melting, freezing Example Of Condensation Vaporization What mass of methanol vapor condenses to a liquid as 20.0 kj of heat are. This process, called vaporization or evaporation, generates a vapor pressure above the liquid. During condensation, a substance is changed from the gaseous to the liquid state of matter. Molecules in the gas phase can collide with the liquid surface and reenter the liquid via. Condensation,. Example Of Condensation Vaporization.
From www.researchgate.net
Vaporization and condensation at a liquidvapor interface (after Moody Example Of Condensation Vaporization A substance condenses when the pressure exerted by its vapour. During condensation, a substance is changed from the gaseous to the liquid state of matter. Molecules in the gas phase can collide with the liquid surface and reenter the liquid via. Vaporization, which is more often referred to as boiling, is the. Condensation is the process in which molecules of. Example Of Condensation Vaporization.
From www.tec-science.com
Specific latent heat of condensation tecscience Example Of Condensation Vaporization A substance condenses when the pressure exerted by its vapour. Condensation is the process in which molecules of a gas slow down, come together, and form a liquid. The molar heat of condensation \(\left( \delta h_\text{cond} \right)\) is the heat released by one mole of a substance as it is converted from a. During condensation, a substance is changed from. Example Of Condensation Vaporization.
From www.exampleslab.com
15 Vaporization Examples Examples Lab Example Of Condensation Vaporization During condensation, a substance is changed from the gaseous to the liquid state of matter. Molecules in the gas phase can collide with the liquid surface and reenter the liquid via. What mass of methanol vapor condenses to a liquid as 20.0 kj of heat are. Condensation is the process in which molecules of a gas slow down, come together,. Example Of Condensation Vaporization.
From www.youtube.com
Changing states of matter 🔁 Melting, Freezing, Evaporation Example Of Condensation Vaporization Molecules in the gas phase can collide with the liquid surface and reenter the liquid via. This process, called vaporization or evaporation, generates a vapor pressure above the liquid. Condensation is the process in which molecules of a gas slow down, come together, and form a liquid. Vaporization, which is more often referred to as boiling, is the. The molar. Example Of Condensation Vaporization.
From www.slideserve.com
PPT States of Matter PowerPoint Presentation, free download ID5735591 Example Of Condensation Vaporization This process, called vaporization or evaporation, generates a vapor pressure above the liquid. The molar heat of condensation \(\left( \delta h_\text{cond} \right)\) is the heat released by one mole of a substance as it is converted from a. A substance condenses when the pressure exerted by its vapour. Condensation is the process in which molecules of a gas slow down,. Example Of Condensation Vaporization.
From www.slideserve.com
PPT Phas e Cha nges PowerPoint Presentation, free download ID6554404 Example Of Condensation Vaporization During condensation, a substance is changed from the gaseous to the liquid state of matter. The molar heat of condensation \(\left( \delta h_\text{cond} \right)\) is the heat released by one mole of a substance as it is converted from a. A substance condenses when the pressure exerted by its vapour. This process, called vaporization or evaporation, generates a vapor pressure. Example Of Condensation Vaporization.
From www.researchgate.net
(a) Schematic figure of one example of the vaporizationcondensation Example Of Condensation Vaporization Condensation, deposition of a liquid or a solid from its vapour, generally upon a surface that is cooler than the adjacent gas. A substance condenses when the pressure exerted by its vapour. Vaporization, which is more often referred to as boiling, is the. The molar heat of condensation \(\left( \delta h_\text{cond} \right)\) is the heat released by one mole of. Example Of Condensation Vaporization.
From www.slideserve.com
PPT UNIT 1 Foundations PowerPoint Presentation, free download ID Example Of Condensation Vaporization The molar heat of condensation \(\left( \delta h_\text{cond} \right)\) is the heat released by one mole of a substance as it is converted from a. This process, called vaporization or evaporation, generates a vapor pressure above the liquid. A substance condenses when the pressure exerted by its vapour. What mass of methanol vapor condenses to a liquid as 20.0 kj. Example Of Condensation Vaporization.
From slideplayer.com
Do now Turn in Phases of Matter homework from Thursday. ppt download Example Of Condensation Vaporization Condensation, deposition of a liquid or a solid from its vapour, generally upon a surface that is cooler than the adjacent gas. Molecules in the gas phase can collide with the liquid surface and reenter the liquid via. This process, called vaporization or evaporation, generates a vapor pressure above the liquid. What mass of methanol vapor condenses to a liquid. Example Of Condensation Vaporization.
From slideplayer.com
Solids and Liquids Chapter 14 Chem B. ppt download Example Of Condensation Vaporization What mass of methanol vapor condenses to a liquid as 20.0 kj of heat are. Condensation, deposition of a liquid or a solid from its vapour, generally upon a surface that is cooler than the adjacent gas. Condensation is the process in which molecules of a gas slow down, come together, and form a liquid. Vaporization, which is more often. Example Of Condensation Vaporization.
From www.slideserve.com
PPT Boiling Point Notes PowerPoint Presentation, free download ID Example Of Condensation Vaporization What mass of methanol vapor condenses to a liquid as 20.0 kj of heat are. The molar heat of condensation \(\left( \delta h_\text{cond} \right)\) is the heat released by one mole of a substance as it is converted from a. During condensation, a substance is changed from the gaseous to the liquid state of matter. This process, called vaporization or. Example Of Condensation Vaporization.